|
|
02 Features
Cereal Foods World, Vol. 64, No. 6
DOI: https://doi.org/10.1094/CFW-64-6-0066
Print To PDF
DisplayTitle Genomics and Gene-Editing Technologies Accelerating Grain Product Innovation
Affiliations
© 2019 Cereals & Grains Association
Abstract
CFWAbstract Rapid advances in genomics technology have continued over the last few years. The ability to edit plant genes has been enabled by the development of clustered regularly interspaced short palindromic repeats and associated protein 9 (CRISPR/Cas9) and related technologies. The continuing improvements in DNA sequencing technology have complemented these new technologies by facilitating efficient and very specific targeting of genetic changes to specific genes and traits. Plant breeding can now move beyond marker-assisted breeding and genetic modification technologies to more complete and planned genetic improvements. Grain crops, in particular, are likely to benefit from increased rates of genetic gain. The genetic basis of grain characteristics will be more easily determined, resulting in new opportunities to improve grain quality and innovate in the development of new traits. Product traceability and identity preservation will also be enhanced by these technologies.
Trying to reach content?
View Full Article
if you don't have access, become a member
Page Content Advances in DNA Sequencing
Advances in genomics are being driven by ongoing developments in DNA sequencing technology (5). The rapid changes in this technology have resulted in a collapse in the cost of DNA sequencing, to the point that lower costs are making it attractive to consider obtaining a DNA sequence of any or all samples of grain that might be used to breed new varieties or that might need intellectual property (IP) protection. The most common form of DNA sequencing, as typified by dye sequencing that can be obtained using platforms supplied by Illumina Inc., produces very large numbers of high-quality sequences of short DNA sequence reads (100–150 bp long) from a sample. More recently, major advances have also been made in the ability to obtain much longer DNA sequence reads, with growing lengths ranging from 10,000 to 100,000 bp, on sequencing platforms produced by Pacific Biosciences of California, Inc., as well as advances in long-read sequencing technology supplied by Oxford Nanopore Technologies Limited. Improvements in sequencing technologies are expected to continue to advance. Progress over the last decade has been dramatic, and we should expect that innovations will result in further significant reductions in costs and that genome sequencing will become a routine analytical tool used in cereal chemistry.
Big Data
Genomics generates very large quantities of data, and advances in data storage, transmission, and analysis (computational capacity) have been essential to enable the application of genomics to grain crops on a large scale. This is illustrated by the fact that 1 Tbp (or 1,000 Gbp) of sequence data covers the wheat genome only around 60 times, and we may need to obtain 10–20 times the coverage for each sample to obtain a reliable and accurate measure of the sequence of a genotype in routine resequencing of thousands of samples. Data storage and computational capacity are likely to remain key constraints to progress in this field.
Genome Assembly
The improvements in DNA sequencing technology have greatly reduced the difficulty of obtaining the DNA sequence of the genome of any organism. The greater accuracy and quantity of data that can be obtained cost effectively, combined with the availability of much longer sequence reads, has made assembly of the genome from sequencing data much easier. Several tools have emerged to support assembly to the chromosome level. The sequencing and de novo assembly of the genome of a grain crop was once a massive effort, but the task is rapidly being simplified. Because we now have good reference genome sequences for most species, resequencing (without the challenges of data assembly) is all that is required.
Resequencing
Once a reference genome sequence is produced for a species, the DNA sequence of any individual can be analyzed by comparison with the reference without the need for de novo assembly. This allows randomly sequenced short DNA sequences from the sample to be aligned with the reference sequence, and all differences can be identified. The result is that for each sample we obtain a complete set of all variations in the DNA sequence relative to the reference sequence. This includes all of the individual sequence variants or single nucleotide polymorphisms and all of the insertions and deletions. Resequencing is becoming a relatively low-cost analysis that provides a large amount of data that defines a sample. This is the ultimate fingerprint of a sample and can be used in IP protection and grain and ingredient identification by industry, as well as in plant breeding. The data also may assist in tracing the origins of food samples, which may become a routine application of this technology. Large-scale applications are being established as well and have the potential to define the genome sequence of all grain varieties in commercial use in the not too distant future. New varieties are likely to be accompanied by a genome sequence for use in IP protection.
Advances in Transcriptomics
Advances in DNA sequencing also are impacting analysis of gene expression, with long-read sequencing technology providing a tool for determination in a single analysis of the complete full-length sequences of all the genes expressed in a plant. This has proven especially useful in plants like wheat (13) that have complex genomes. The variation in gene expression in different tissues (e.g., different parts of the grain) at different stages (e.g., grain development) and in different genotypes can be assessed quantitatively using a technique known as RNA sequencing (RNA-Seq).
RNA-Seq
RNA-Seq uses short-read sequencing technology to determine the frequency of gene expression of all the genes in a sample and has been used to identify many genes associated with different grain quality characteristics. This technique has revealed a gene associated with bread quality, more specifically loaf volume (4), and identified the genetic basis of variation in the milling performance (flour yield) (11) of wheat genotypes.
Bulked DNA Sequencing
The genetic basis of grain quality traits can be discovered by sequencing bulked DNA from plants that differ for the trait of interest. For example, the genetic control of amylose content in rice was determined by sequencing the combined DNA of 10 genotypes with high amylose contents and comparing the whole genome sequence with the bulked DNA of 10 genotypes with low amylose contents. This bulk sequencing identified both the genes encoding the relevant starch biosynthesis genes and those regulating the expression of starch genes. A similar approach could be utilized to analyze any traits that show variation in a population of grains.
Genome Manipulation
Genomics is being used to reveal the molecular basis of many important grain quality traits and provide tools for their selection in breeding. This has resulted in the wider use of molecular tools such as genetic markers in the breeding and selection of new varieties. The increasing knowledge of grain species genomes also supports more direct manipulation of the genetics of a grain crop, as has been practiced in producing plants that are considered to be genetically modified organisms (GMOs) because foreign DNA has been inserted. New techniques, known generally as gene-editing technologies, are now finding applications in grain crops. Several different technologies have been developed for gene editing. The best known of these is clustered regularly interspaced short palindromic repeats and associated protein 9 (CRISPR/Cas9)-based gene editing (16).
This technique is used to make a precise cut in the DNA at a target location. The DNA naturally repairs itself, resulting in a mutation. New sequences can also be inserted at this point by guiding the repair with synthetic DNA introduced into the cell at the same time.
This technology makes it possible to mutate any gene in a highly targeted way. The precision of the method relies on the knowledge of the plant genome. Having a precise DNA sequence to work with facilitates the design of the experiment to target only one site in the genome or multiple sites, depending on the objective of the modification. The availability of techniques for cost-effective sequencing of the whole genome makes it possible to ensure that the gene editing is targeted and not likely to affect off-target genes. Sequencing technology also provides a tool for verifying that the genetic changes have been targeted correctly.
Regulation
Gene editing, especially the use of this technology to disable or knock out a gene, is not considered to achieve any outcome that could not occur at much lower frequency and with more nontarget effects in conventional mutation breeding. Because of this, in some countries gene editing in which the DNA repair is not guided is not considered to generate a GMO. Current European law, as interpreted by the courts, classifies this type of material as a GMO, while in Australia regulations enacted as of October 2019 clarify that this type of material is not a GMO. The global regulatory environment for the technology in the medium and long term remains uncertain.
Modification of the structure and amount of starch, cell walls, protein, and lipids in grains are all potential applications for this technology. The resulting products may not be considered GMOs outside of Europe under current regulations. Widespread successful adoption of this technology in the near future might challenge European authorities to update their regulatory system. The response of policy makers globally will be critical in determining the extent to which this technology will become a path to major innovations in food products.
Examples of New Opportunities Offered by Gene Editing
 Gene-editing technology delivers a powerful new tool for plant breeding and grain product innovation. The technology has been widely applied to rice due to the extensive genomic resources and transformation systems available for this grain. Editing has been used to establish the precise roles of genes by examining the effects of their downregulation. Rice traits (Table I) that have been successfully manipulated include grain size (9), fragrance (9), amylose content (15), and shelf life (9). All of these edits involve the very straightforward application of CRISPR to downregulate genes controlling these traits. The addition of the fragrance trait to rice is a great example of potential routine applications for this technology. It was established some time ago that this trait, which adds significant value to rice, is controlled by a single gene (2). Fragrance is associated with loss of function of the gene (1), making this an ideal trait to manipulate by knock out using gene editing. We can now add fragrance to any rice variety by simply editing out this one gene. The application of this technology to more difficult gene-editing tasks, such as addition of new genes or precise replacement of genes (7), can be expected to follow but is likely to face a more difficult regulatory path in some markets. Gene-editing technology delivers a powerful new tool for plant breeding and grain product innovation. The technology has been widely applied to rice due to the extensive genomic resources and transformation systems available for this grain. Editing has been used to establish the precise roles of genes by examining the effects of their downregulation. Rice traits (Table I) that have been successfully manipulated include grain size (9), fragrance (9), amylose content (15), and shelf life (9). All of these edits involve the very straightforward application of CRISPR to downregulate genes controlling these traits. The addition of the fragrance trait to rice is a great example of potential routine applications for this technology. It was established some time ago that this trait, which adds significant value to rice, is controlled by a single gene (2). Fragrance is associated with loss of function of the gene (1), making this an ideal trait to manipulate by knock out using gene editing. We can now add fragrance to any rice variety by simply editing out this one gene. The application of this technology to more difficult gene-editing tasks, such as addition of new genes or precise replacement of genes (7), can be expected to follow but is likely to face a more difficult regulatory path in some markets.
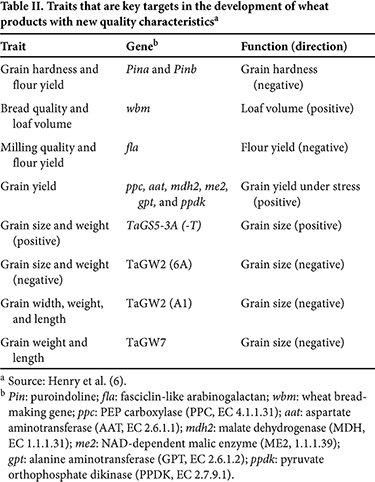 Despite the genetic complexity of hexaploid wheat, CRISPR is being applied successfully to the development of new wheat types. Gene editing may be designed to specifically target one copy of a gene in the hexaploid genome or to target all copies across the subgenomes. Many wheat genes that control quality traits have been identified (Table II) and could be manipulated using this technology. Low-gluten wheat has been developed using gene editing (10,14) and is a good example of the type of novel trait that can be expected from the application of CRISPR to agricultural and food products. This technology has also been applied to other grain species such as maize (corn) (3) and legumes (8). Despite the genetic complexity of hexaploid wheat, CRISPR is being applied successfully to the development of new wheat types. Gene editing may be designed to specifically target one copy of a gene in the hexaploid genome or to target all copies across the subgenomes. Many wheat genes that control quality traits have been identified (Table II) and could be manipulated using this technology. Low-gluten wheat has been developed using gene editing (10,14) and is a good example of the type of novel trait that can be expected from the application of CRISPR to agricultural and food products. This technology has also been applied to other grain species such as maize (corn) (3) and legumes (8).
The downregulation of large numbers of genes has been demonstrated, making the modification of large families of genes a feasible target in gene editing. This suggests that radical, large-scale redesign of grains may be possible, which may make it possible to consider transfer of end uses between grains. For example, rice with breadmaking qualities or wheat designed to be consumed as a whole grain product like rice could be developed. A wide range of nutritional and functional modifications are likely to be possible.
Future Directions
The grains of the future can now be conceived and developed with a composition and quality designed for specific nutritional, processing, and end-use functionalities in food products. The approaches used today, which involve molecular marker- or genomic selection-assisted breeding, largely deliver higher yielding variants of conventional types of grain. New data-driven approaches will increasingly be applied to product design and construction (12). These technologies should accelerate the rate of development of new grain products with novel, market-disruptive traits. Grain production efficiency, both on-farm and in processing, will be enhanced by the application of these powerful genetic tools to improve grain quality traits, with the potential for some step changes to boost yields beyond those being delivered by more incremental quantitative genetics approaches. These new breeding technologies may remove the constraint of quality selection in breeding and enable a significant increase in the rate of genetic gain in yield for grain crops. Consumers, however, will be more likely to notice the availability of grain products with new quality attributes.
 Robert Henry conducts research on the development of new products from plants. He is a professor of innovation in agriculture and foundation director of the Queensland Alliance for Agriculture and Food Innovation (QAAFI), a research institute of the University of Queensland established in collaboration with the Queensland government. He was previously director of the Centre for Plant Conservation Genetics at Southern Cross University, research director of the Grain Foods CRC, and research program leader in the Queensland Agricultural Biotechnology Centre. His current research targets plant genome sequencing for the capture of novel genetic resources for diversification of food crops to deliver improved food products. Robert Henry conducts research on the development of new products from plants. He is a professor of innovation in agriculture and foundation director of the Queensland Alliance for Agriculture and Food Innovation (QAAFI), a research institute of the University of Queensland established in collaboration with the Queensland government. He was previously director of the Centre for Plant Conservation Genetics at Southern Cross University, research director of the Grain Foods CRC, and research program leader in the Queensland Agricultural Biotechnology Centre. His current research targets plant genome sequencing for the capture of novel genetic resources for diversification of food crops to deliver improved food products.
References References
- Bradbury, L., Gillies, S., Brushett, D., Waters, D., and Henry, R. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 68:439, 2008.
- Bradbury, L. M. T., Fitzgerald, T. L., Henry, R. J., Jin, Q., and Waters, D. L. E. The gene for fragrance in rice. Plant Biotechnol. J. 3:363, 2005.
- Feng, C., Yuan, J., Wang, R., Liu, Y., Birchler, J. A., and Han, F. P. Efficient targeted genome modification in maize using CRISPR/Cas9 system. J. Genet. Genomics 43:37, 2016.
- Furtado, A., Bundock, P. C., Banks, P. M., Fox, G., Yin, X., and Henry, R. J. A novel highly differentially expressed gene in wheat endosperm associated with bread quality. Sci. Rep. 5:10446, 2015.
- Henry, R. J. Next-generation sequencing for understanding and accelerating crop domestication. Briefings Funct. Genomics 11:51, 2012.
- Henry, R. J., Furtado, A., and Rangan, P. Wheat seed transcriptome reveals genes controlling key traits for human preference and crop adaptation. Curr. Opin. Plant Biol. 45:231, 2018.
- Li, S. Y., Li, J. Y., He, Y. B., Xu, M. L., Zhang, J. H., Du, W. M., Zhao, Y. D., and Xia, L. Q. Precise gene replacement in rice by RNA transcript-templated homologous recombination. Nat. Biotechnol. 37:445, 2019.
- Meng, Y. Y., Hou, Y. L., Wang, H., Ji, R. H., Liu, B., Wen, J. Q., Niu, L. F., and Lin, H. Targeted mutagenesis by CRISPR/Cas9 system in the model legume Medicago truncatula. Plant Cell Rep. 36:371, 2017.
- Mishra, R., Joshi, R. K., and Zhao, K. Genome editing in rice: Recent advances, challenges, and future implications. Front. Plant Sci. DOI: https://doi.org/10.3389/fpls.2018.01361. 2018.
- Moehs, C. P., Austill, W. J., Holm, A., Large, T. A. G., Loeffler, D., et al. Development of reduced gluten wheat enabled by determination of the genetic basis of the lys3a low hordein barley mutant. bioRxiv. DOI: https://doi.org/10.1101/354548. 2018.
- Nirmal, R. C., Furtado, A., Rangan, P., and Henry, R. J. Fasciclin-like arabinogalactan protein gene expression is associated with yield of flour in the milling of wheat. Sci. Rep. DOI: https://doi.org/10.1038/s41598-017-12845-y. 2017.
- Pouvreau, B., Vanhercke, T., and Singh, S. From plant metabolic engineering to plant synthetic biology: The evolution of the design/build/test/learn cycle. Plant Sci. 273:3, 2018.
- Rangan, P., Furtado, A., and Henry, R. J. The transcriptome of the developing grain: A resource for understanding seed development and the molecular control of the functional and nutritional properties of wheat. BMC Genomics 18:766, 2017.
- Sánchez-León, S., Gil-Humanes, J., Ozuna, C. V., Giménez, M. J., Sousa, C., Voytas, D. F., and Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 16:902, 2018.
- Sun, Y., Jiao, G., Liu, Z., Zhang, X., Li, J., et al. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. DOI: https://doi.org/10.3389/fpls.2017.00298. 2017.
- van der Oost, J. New tool for genome surgery. Science 339:768, 2013.
|